Using centrifugal force to decelerate particles creates new opportunities for chemistry and quantum information processing
Compared with our breath, passenger planes move at a pretty leisurely pace. On the average, nitrogen molecules, for example, travel at a speed of more than 1,700 kilometres per hour at room temperature, or almost one-and-a-half times the speed of sound. This means the particles are much too fast for many experiments, and also some conceivable applications. However, physicists at the Max Planck Institute of Quantum Optics in Garching have now found a rather simple way to slow down polar molecules to about 70 kilometres per hour. They let the molecules of various substances, such as fluoromethane, run up against the centrifugal force on a rotating disk, while being guided by electrodes. The speed of the decelerated molecules corresponds to a temperature of minus 272 degrees Celsius. The new method makes it possible to produce relatively large quantities of cold molecules in a continuous flow, which could be useful, for instance, for targeted chemical reactions of individual particles, or the processing of quantum information.
 Zoom Image
Zoom Image
Deceleration in the centrifuge: Molecules lose speed drastically when they are guided against the centrifugal force to... [more]
© MPI of Quantum Optics
Chemical reactions are pretty uncontrolled. The reaction partners encounter each other by chance and then collide quite violently, whereupon it is not certain they will do what chemists expect them to do. Bringing them close to each other systematically and at a leisurely pace could favour some transformations that otherwise rarely occur. For this to happen, chemists need slow, and therefore cold, molecules, and they need these in large quantities. Physicists as well rely on cold molecules for many experiments, as well as for new technological applications, such as quantum information processing. For many scientists, especially in low-temperature physics, it should thus be welcome news that researchers working with Sotir Chervenkov and Gerhard Rempe at the Max Planck Institute of Quantum Optics have developed a versatile and efficient brake for polar molecules.
The Garching-based team’s decelerator slows down the particles – in their current experiments, molecules of fluoromethane, trifluoromethane and 3,3,3-trifluoropropine – from about 700 to 70 kilometres per hour. Since the speed of the particles can be expressed in temperature units, this corresponds to reducing the temperature from 100 K to 1 K, or from minus 173 to minus 272 degrees Celsius. “Nitrogen-cooled sources supply molecules at 100 Kelvin, and we also know some good methods for further cooling molecules at 1 Kelvin,” says Sotir Chervenkov. “But there are currently no efficient methods for the range in between, and particularly none that produce a continuous flow of cold molecules.”
Four electrodes guide molecules to the centre of the centrifuge
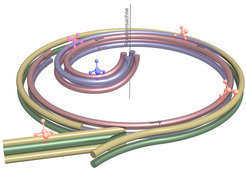
The Max Planck researchers rely here on an amply known force, but one that has never before been used to slow down molecules: centrifugal force. The molecular brake thus consists of a centrifuge that rotates at up to 43 revolutions per second: a 40-centimetre-in-diameter rotating disk on which the particles are guided from its periphery to its centre. Four electrodes with alternating polarity spaced one millimetre apart and arranged at the apices of a square serve as guiderails imposing with their electric field a travel direction on the molecules.
 Zoom Image
Zoom Image
The principle of the molecular brake: Four electrodes initially guide polar molecules from the entry of the centrifuge... [more]
© Sotir Chervenkov/MPI of Quantum Optics
Two static electrodes gird the disk brake. Through an opening in this double ring, the Max Planck physicists guide the particles into the decelerator. On the disk are likewise mounted, along almost the entire circumference, two electrodes, but not forming closed rings. Rather, the two electrodes bend in a spiral toward the centre across about a quarter of the circular area.
To ensure that there are always four electrostatic guiderails keeping the molecules on track along their deceleration path, a further electrode pair accompanies the particles along the spiral coil. These electrodes are tapered and interface with the static electrode ring at a distance of just 0.2 millimetres, so that it looks as if they branched out of the ring. The molecules are thus moved smoothly onto the curved path, on which they fight against the centrifugal force and drastically lose speed until a further curve in the electrodes in the centre of the disk guides them up and away from the decelerator.
Molecules would have to fly up 2,000 metres against the Earth’s gravitational field
“The deceleration is accomplished in two steps,” explains Martin Zeppenfeld, who originally devised the concept of the molecular brake. “Initially, the molecules slow down when they pass from the laboratory system to the rotating system.” This is comparable to a father running along next to his child on a rotating carousel. He moves with respect to the environment, but for the child, he’s not moving.
“Additionally, the molecules are exposed to the outwardly directed centrifugal force,” adds Martin Zeppenfeld. “On their way to the centre, the particles must surmount a huge mountain, and are continuously decelerated while doing so, until they finally come almost to a standstill.” For comparison: for the particles to experience the same braking effect in the Earth’s gravitational field, they would have to fly 2,000 meters upward.
Some of the methods currently used to decelerate polar molecules use electrodes not only as guiderails, but also as the actual brake. However, with practicable field strengths, the braking effect remains low, requiring that the particles be sent repeatedly to this electrical potential mountain. This not only results in many particles being lost, but they also don’t leave the decelerator in a continuous flow, but rather in the form of particle pulses, or in other words, in batches.
Centrifuge deceleration is versatile and easy to use
“What is new about our centrifuge deceleration is its continuous operation, the large number of molecules in the resulting beams, its application versatility, and its relative ease of handling,” says Gerhard Rempe, Director at the Max Planck Institute of Quantum Optics. In principle, atoms or neutrons can also be decelerated by a centrifugal force. However, these particles aren’t polar and therefore can’t be guided through the centrifuge using an electric field.
The researchers in Garching now want to further cool the centrifuge-decelerated molecules. They aim to do this using Sisyphus cooling, which they just recently developed, and which is suitable for molecules that are already very cold. Here, an electric field decelerates the optically excited molecules. Through a combination of both methods, the researchers obtain a sufficiently dense flow of extremely cold molecules, allowing them to steer them toward one another to create specific collisions and control their chemical reaction. But the extremely cold molecules could also be accumulated to form clouds that could serve as the register of a quantum computer that is particularly fast for certain arithmetic operations. Thus, the closed cold chain for particles opens up completely new perspectives for chemistry and physics.
Source: http://www.mpg.de/7874908/centrifugal-forces-molecules-brakes