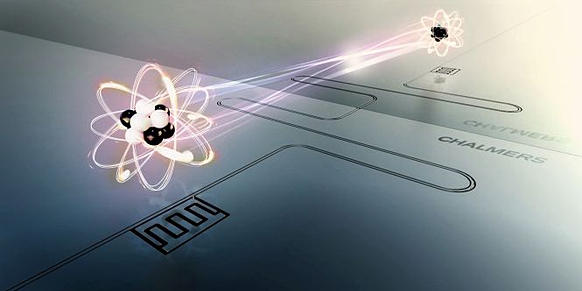
Resonators are an important tool in physics. The curved mirrors inside the resonators usually focus light waves that act, for instance, on atoms. Physicists at ETH Zurich have now managed to build a resonator for electrons and to direct the standing waves thus created onto an artificial atom.
More than two thousand years ago the Greek inventor and philosopher Archimedes already came up with the idea of using a curved mirror to reflect light in such a way as to focus it into a point – legend has it that he used this technique to set fire to the ships of the Roman enemies. Today such curved or parabolic mirrors are used in a host of technical applications ranging from satellite dishes to laser resonators, where light waves are amplified between two mirrors. Modern quantum physics also makes use of resonators with curved mirrors. In order to study single atoms, for example, researchers use the light focused by the mirrors to enhance the interaction between the light waves and the atoms. A team of physicists at ETH Zurich, working within the framework of the National Centre of Competence in Research Quantum Science and Technology (NCCR QSIT), have now managed to build a resonator that focuses electrons rather than light waves. In the near future, such resonators could be used for constructing quantum computers and for investigating many-body effects in solids.
In their experiments the post-doctoral researchers Clemens Rössler and Oded Zilberberg used semiconductor structures in which electrons are free to move only in a single plane. At one end of that plane there is a so-called quantum dot: a tiny trap for electrons, only a hundred nanometers wide, in which owing to quantum mechanics the electrons exist in well-defined energy states similar to those of an atom. Such quantum dots are, therefore, also known as “artificial atoms”. At the other end, just a few micrometers away, a bent electrode acts as a curved mirror that reflects electrons when a voltage is applied to it.
Better materials
The possibility to focus electrons in this way was already investigated in 1997 at Harvard University. The ETH researchers, however, were now able to work with much better materials, which were produced in-house in Werner Wegscheider’s laboratory for Advanced Semiconductor Quantum Materials. “These materials are a hundred times cleaner than those used at the time”, explains Rössler, “and consequently the electrons can move undisturbed a hundred times longer.”
This, in turn, allows the quantum mechanical wave nature of the electrons to become very clearly visible, which was not the case in those earlier works.
In their experiments, the physicists detect this wave nature by measuring the current flowing from the quantum dot to the curved mirror. This current changes in a characteristic way as the applied voltage is varied. “Our results show that the electrons in the resonator do not just fly back and forth, but actually form a standing wave and thus couple coherently to the quantum dot”, stresses Rössler, who developed the experiment in the group of ETH professor Klaus Ensslin. Differently from light waves, the spin of the electrons also causes them to behave as tiny magnets. Indeed, the researchers were able to show that the interaction between the electrons in the quantum dot and the electronic wave in the resonator happens through the spin. “In the future, this spin-coherent coupling could make it possible to connect quantum dots over large distances”, says Zilberberg, who has developed a theoretical model for Rössler’s experiment in the group of ETH professor Gianni Blatter.
Suitable for quantum computers
For some time now, quantum dots have been considered as possible candidates for making so-called quantum-bits or "qubits", which are used in quantum computers. Until now the quantum dots in such a computer needed to be very close to each other in order to achieve the necessary coupling for performing calculations. This, however, made it difficult to control and read out individual qubits. A long-distance coupling through an appropriately designed resonator could elegantly solve this problem.
Basic science could also benefit from the electron resonators realized by the ETH researchers, for instance in studies of the Kondo effect. This effect occurs when many electrons together interact with the magnetic moment of an impurity in a material. With the help of a resonator and a quantum dot simulating such an impurity, the physicists hope to be able to study the Kondo effect very precisely.
It took the young post-docs just over a year to go from the idea for their research – which grew out of discussions during a previous experiment – to the paper that has now been published. Zilberberg has a simple explanation for why this could happen so fast: “Within the QSIT network it’s easy to forge spontaneous collaborations across different groups as we are close both thematically and spatially, and we are often involved in common projects anyway. Plus, if one needs the opinion of an expert, there is usually one just down the corridor.”